-
春兰(Cymbidium goeringii)是兰科(Orchidaceae)兰属(Cymbidium)中的一个种,具有较高的观赏价值和经济价值。‘宋梅’是中国春兰传统铭品,为梅瓣花的代表品种,被誉为春兰“四大天王”之首,“春兰之王”等,幽香馥郁,浓而不浊,深受广大兰友的喜爱。
镁原卟啉Ⅸ甲基转移酶(Magnesium protoporphyrin Ⅸ methyltransferase,ChlM)是叶绿素合成过程中的关键酶,也是重要的调控酶,其活性受到光照、叶绿体氧化还原状态和叶片中叶酸含量调控。此外,镁原卟啉Ⅸ甲基转移酶不仅影响PSⅠ、PSⅡ和Cytb6f 蛋白复合体内重要蛋白的积累,参与叶绿体到细胞核的反向信号转导、ABA 信号转导,还通过调控ChlH 表达影响镁螯合酶活性[1]。原卟啉Ⅸ甲基转移酶是单基因产物,定位在叶绿体被膜和类囊体膜上,其发挥作用时依赖于腺苷甲硫氨酸( Ado-Met) ,属于S-腺苷-L-甲硫氨酸依赖的甲基转移酶大家族[2-3]。拟南芥At4g25080是编码该甲基转移酶的基因,在烟草[4]、水稻[5]、豌豆[6]、衣藻[7]中均发现ChlM 序列同源物。本研究从‘宋梅’组培苗叶片中克隆了春兰ClChlM1基因,构建pART-ClChlM1植物表达载体,进而在烟草中瞬时表达鉴定了该基因的叶绿素生物合成功能,旨在为春兰叶绿素合成途径的揭示及春兰叶色变异提供理论依据。
-
供试材料为春兰‘宋梅’组培苗,保存在四川省农业科学院园艺研究所组培室(见图1)。
-
根据天根生化科技有限公司的植物总RNA提取试剂盒说明书,从叶片样品中提取总RNA,利用Nanodrop2000(Thermo Scientific,美国)对RNA的浓度和纯度进行检测,1.5%琼脂糖凝胶电泳检测RNA 完整性。
使用Aidlab公司反转录试剂盒(TUREscript 1st Strand cDNA Synthesis Kit)进行cDNA的合成,反应体系为40 μL:500 ng总RNA,8 μL 5×RT Reaction Mix,2 μL Oligo(dT)引物,2 μL TUREscript H- RTase/RI Mix,采用RNase Free dH2O补足至40 μL。反转录反应程序条件为:42 ℃ 60 min,65 ℃ 10 min。反应结束后,将得到的cDNA放置−20℃保存,用前稀释10倍使用。
-
根据表1的引物,从‘宋梅’cDNA中克隆了ClChlM1基因。PCR扩增程序:96 ℃ 10 min;96℃ 30 s;60 ℃ 30 secs,72 ℃ 1 min,30个循环;72 ℃ 10 min。将扩增产物经1.5%琼脂糖凝胶分离后对目的片段进行切胶回收,连入pMT18-T载体并转化大肠杆菌DH10B过夜培养。次日挑选克隆经菌落PCR验证后送至上海美吉生物医药科技有限公司测序。
基因名称
Name引物序列
Primer sequence用途
UsageClChlM1-F ATGGCTTCTTTCGCCACCGTTC 基因全长扩增
Gene amplificationClChlM1-R TCAGGCGGCCGGGACGAGGA ClChlM1-XhoI-F CCGCTCGAGATGGCTTCTTTCGCCACC 转基因载体构建 ClChlM1-SalI-R ACGCGTCGACTCAGGCGGCCGGGACG Transgenic vectors Construction Kan-F GATCTGGACGAAGAGCATCAG 转基因检测
Transgenic detectionKan-R CTCGTCAAGAAGGCGATAGAAG ClHemA-F CCAAGATGCTCTGGGTTGATAG qRT-PCR ClHemA-R ATGGGATACGGGCTTCAAATAC ClGsa-qF GTTGATGCCAGGAGGTGTAA qRT-PCR ClGsa-qR GCCCTTCACGGAGTCAATAA ClCHLI-qF CTGAACGTCGATGGATTGAGAG qRT-PCR ClCHLI-qR TATCCTCCGGAGTGACCTTATC ClCHLH-qF GGTATTTCTGCCCTTCCATCTAT qRT-PCR ClCHLH-qR GCAGCTCCACATCTCGTAAA ClLHCB-qF GCATTCGCTGAGTTGAAAGTG qRT-PCR ClLHCB-qR GGTCCCTTTCCTGTGACAATAG ClEF1-α-qF ATTGGTGGAATTGGTACTGTCC Reference gene/qRT-PCR ClEF1-α-qR CCGCAACATTCTTGACATTAAA Table 1. Primers used in the present study
-
选取铁皮石斛的DcChlM1、马蹄蝴蝶兰的PeChlM1、油棕的EgChlM1、深圳拟兰的AsChlM1、风铃木的HiChlM1、香蕉的MaChlM1、玉米的ZmChlM1、柑橘的CsChlM1、黄麻的CoChlM1,以及春兰的ClChlM1,采用MEGA 5.0构建系统进化树。
-
设计酶切引物对ClChlM1基因进行扩增,将扩增后的片段经XhoI和SalI双酶切后连入pBI121-GFP载体,并对拟南芥原生质体进行转化。通过荧光显微镜(Olympus BX51)观察融合蛋白的定位。
-
为了获得ClChlM1过量表达载体,将ClChlM1编码区序列插入pART-CAM,获得pART-ClChlM1载体[8]。通过农杆菌介导法将载体导入到本氏烟草中[9]。表1引物检测烟草的阳性转化率。
-
按照Alawady and Grimm的方法检测ALA[4]。取烟草叶片,在含有40 mM乙酰丙酸(pH 6.9)的20 mM磷酸盐缓冲液(pH值7.5)中,置于光下培养4小时。取上清液,加入乙酰乙酸煮沸10分钟。加入等量的Ehrlich’s试剂,在553 nm下检测ALA。
检测烟草的叶绿素含量,用95%的乙醇和稀释的丙酮碾磨叶片。用紫外分光光度计检测665 nm, 649 nm 和 470 nm波长[10]。
-
引物序列详见表1。ACTIN基因作为内参,通过2−△△Ct法对目的基因表达量进行计算。
-
通过RT-PCR获得了ClChlM1的开放阅读框(ORF)。DNA测序结果显示,ClChlM1的ORF长度为945 bp,编码314个氨基酸残基组成的蛋白质(见图2)。将ClChlM1序列提交至NCBI,序列号为MG574594。
-
选取春兰的ClChlM1、铁皮石斛的DcChlM1、深圳拟兰的AsChlM1、油棕的EgChlM1进行氨基酸序列比对,结果表明春兰的ClChlM与其余四种有较高的同源性,且都含有守的Mg-por_mtran_C区域(见图3 A)。
为进一步分析ClChlM进化关系,选取铁皮石斛的DcChlM1、马蹄蝴蝶兰的PeChlM1、油棕的EgChlM1、深圳拟兰的AsChlM1、风铃木的HiChlM1、香蕉的MaChlM1、玉米的ZmChlM1、柑橘的CsChlM1、黄麻的CoChlM1蛋白序列进行进化树构建,结果显示植物ChlM蛋白分为三个亚类,其中ClChlM与DcChlM1、PeChlM1、AsChlM1、EgChlM1、MaChlM1属于第I亚类,且与DcChlM1亲缘关系最近(见图3 B)。
-
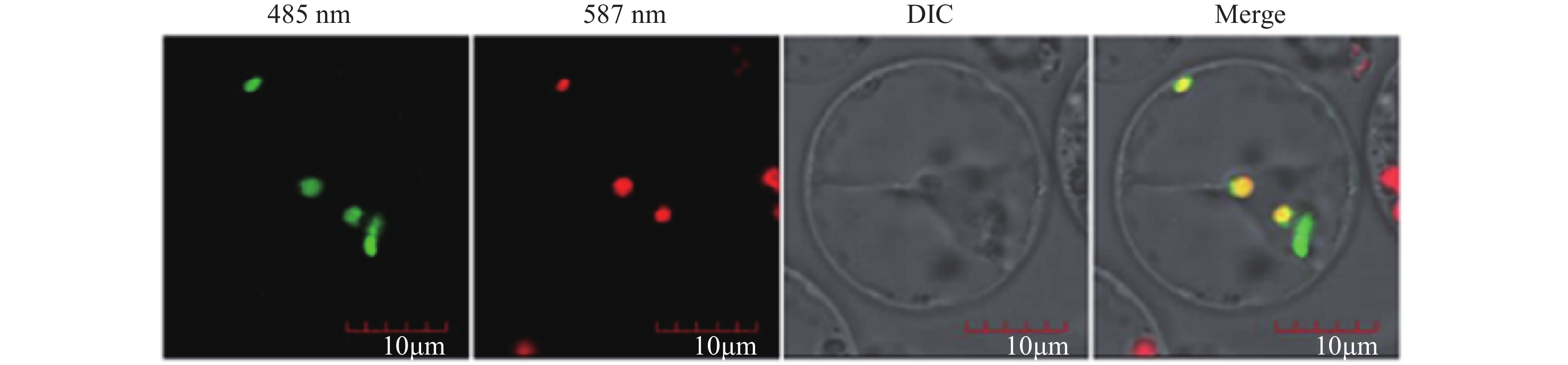
原生质体转化结果显示ClChlM1-GFP融合蛋白绿色荧光与叶绿体探针红色荧光融合为黄色荧光,表明ClChlM1定位于叶绿体(见图4)。
-
为进一步验证ClChlM1基因的功能,采用叶盘法将其转化到烟草中(见图5)。经PCR检测,获得三个转基因株系(见图5E)。
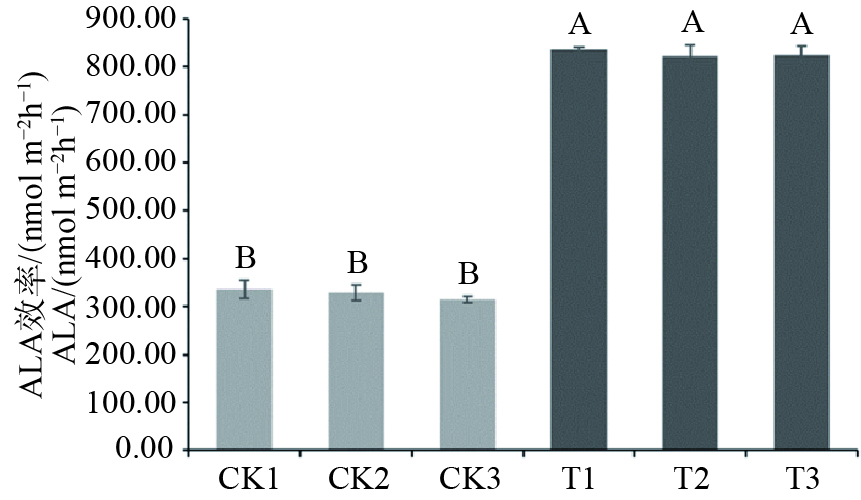
叶绿素是一种含卟啉化合物,ALA是卟啉生物合成过程中的一个重要前体。测定结果显示转基因株系中ALA合成效率显著高于对照株系,约为对照株系中的2.28倍(见图6)。
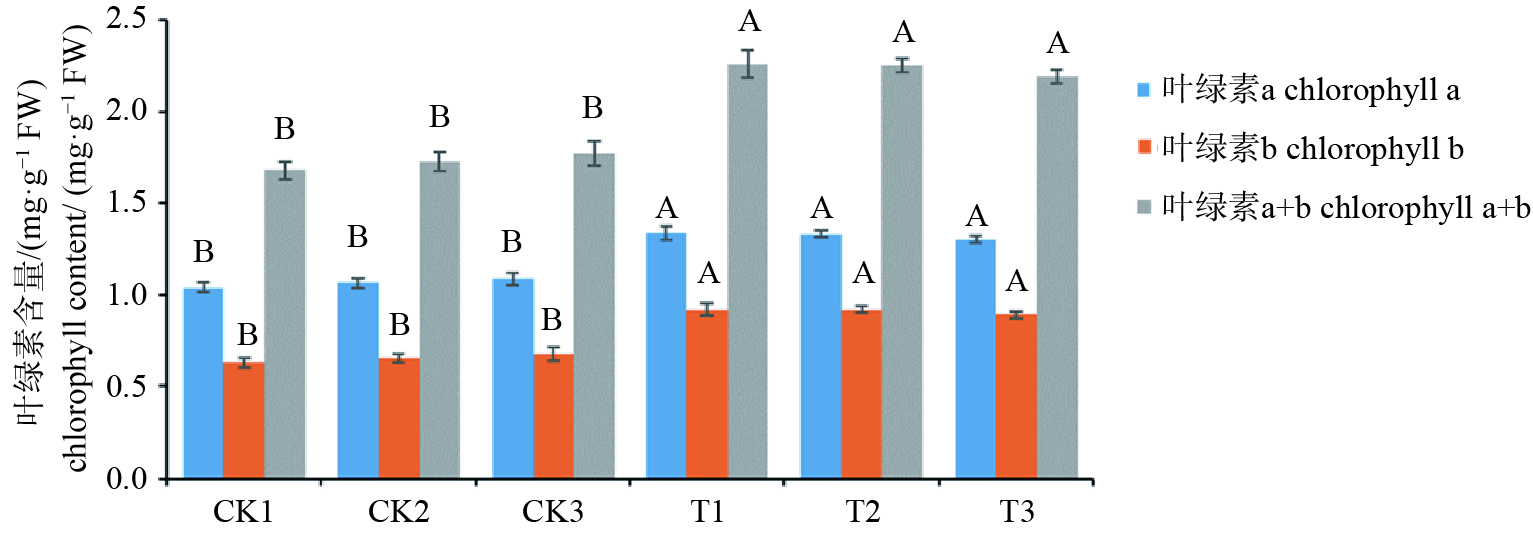
叶绿素含量测定结果显示转基因株系中叶绿素a、叶绿素b、叶绿素总量含量远高于对照株系,分别是对照株系的1.32倍、1.56倍、1.35倍(见图7)。
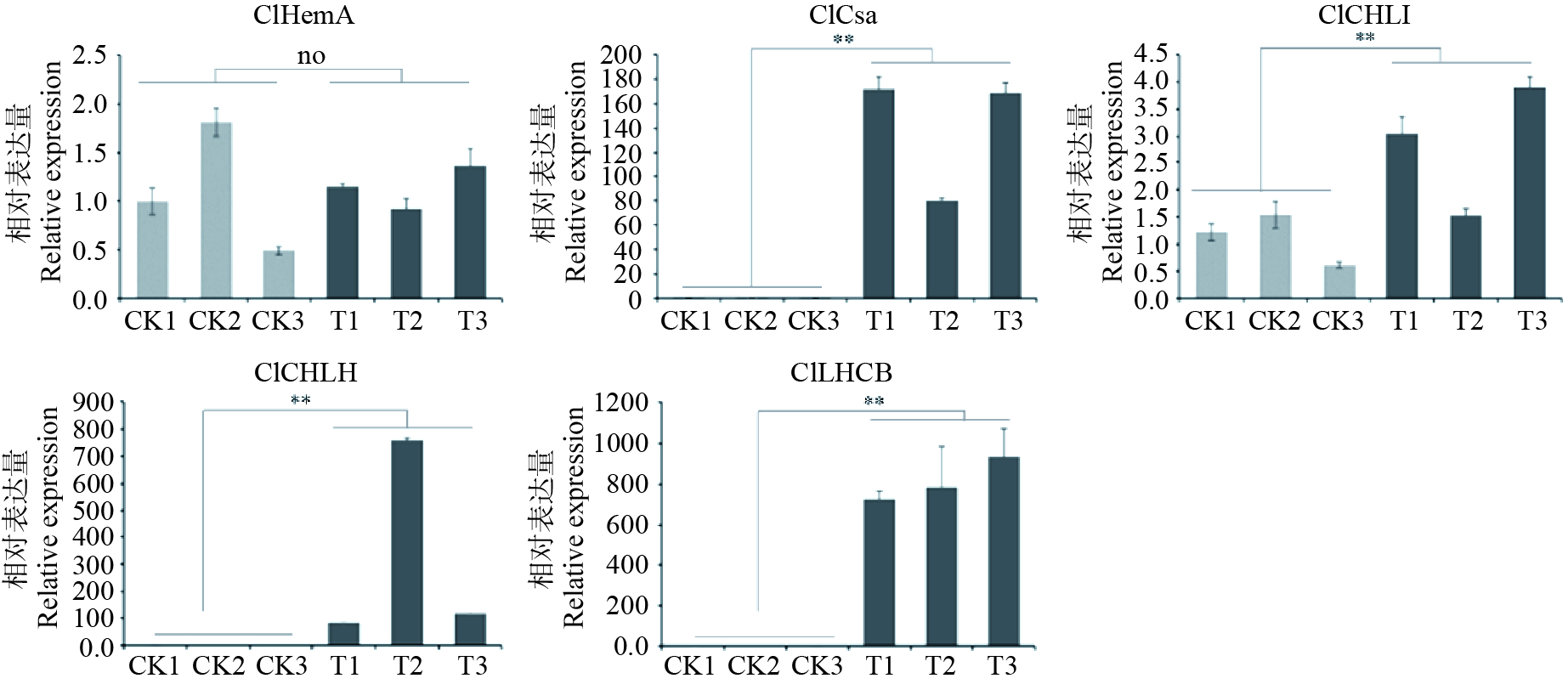
进一步对光合作用相关成员谷氨酰tRNA还原酶 (HemA), 谷氨酸酯-1-半醛2,1-氨基变位酶 (Gsa), 叶绿素a/b结合蛋白(LHCB), 镁离子螯合酶CHLI和镁离子螯合酶CHLH在转基因烟草中的表达模式进行分析,结果显示除了ClHemA外,ClGsa、ClLHCB、ClCHLI、ClCHLH在转基因株系中的表达量均显著高于对照组(见图8)。
-
从春兰组培苗中克隆的ClChlM1基因ORF长度为945 bp,氨基酸序列分析显示ClChlM1拥有植物ChlM蛋白高度保守的Mg-por_mtran_C结构域,进化树分析显示ClChlM1与同为兰科植物的铁皮石斛的DcChlM1亲缘关系最近。
高等植物叶绿素生物合成属于典型的卟啉代谢途径,由谷氨肽-tRNA到叶绿素a和叶绿素b的形成共需经历十余步酶促反应,其中催化镁原卟啉Ⅸ(MgP)形成镁原卟啉Ⅸ单甲醚(MgPME)的ChlM是该过程关键酶之一[12]。最近还有研究表明ChlM可以在转录后水平对光合作用编码蛋白进行调控。LHCB是捕光天线蛋白,位于叶绿体类囊体膜上,作用是将吸收的光能传递到作用中心,启动光合作用。在大麦中,Gadjieva等发现ChlM产物MgPME的积累会促进LHCB基因的表达[13-14]。在本研究中,除了ClLHCB外,还发现ClGsa, ClCHLI和ClCHLH在ClChlM1过表达植株中显著上调,而ClHemA基因的表达量则无变化。HemA编码谷氨肽-tRNA还原酶,是叶绿素生物合成途径第一个酶;Gsa编码的谷氨酸-1-半醛转氨酶是叶绿素生物合成途径第二个酶,主要负责ALA的合成;CHLI和CHLH编码的蛋白是镁螯合酶的不同亚基,主要负责镁原卟啉IX的合成。因此,推测ClChlM1可反馈调节叶绿素的生物合成,具体的调控机制将是下一步研究重点。
Molecular Cloning and Functional Analysis of Magnesium Protoporphyrin Ⅸ Methyltransferase Gene in Tissue Culture Seedlings of Cymbidium goeringii
doi: 10.12172/202110090002
- Received Date: 2021-10-09
- Available Online: 2022-06-27
- Publish Date: 2022-08-23
-
Key words:
- Cymbidium goeringii. /
- Tissue culture /
- Chlorophyll /
- Magnesium protoporphyrin Ⅸ methyltransferase gene
Abstract: The magnesium protoporphyrin IX methyltransferase (ChlM) is one of the key rate-limiting enzymes in chlorophyll synthesis and development of chloroplasts, which plays an important role in plant growth and development. To better understand the role of ChlM in Cymbidium goeringii, ChlM gene was cloned from the tissue culture seedling of Cymbidium goeringii ‘Song Mei’. The full-length open reading frame of ClChlM1 was 945 bp and encoded 314 amino acids. The sequence alignment showed that ClChlM1 contained an Mg-por_mtran_C domain, and subcellular location analysis indicated ClChlM1 was located in chloroplast. Furthermore, the overexpression of ClChlM1 in tobacco leaves could significantly increase the content of chlorophyll and ALA compared with wild type tobacco leaves. In addition, the leaf area of overexpressed tobacco was larger than that of wild type. In the over-expressed transgenic lines, the gene expression of glutamate -1- semialdehyde 2,1- aminomutase (Gsa), chlorophyll a/b binding protein (LHCB), magnesium chelatase CHLI and magnesium chelatase CHLH were up-regulated.



























 DownLoad:
DownLoad: