-
九寨沟国家级自然保护区位于中国西南部的四川省,处于湿润的四川盆地边缘向半干旱的青藏高原延伸的过渡地带。它是联合国列入的世界自然遗产和人与生物圈保护区[1]。九寨沟作为世界生物多样性热点之一,具有保护全球生物多样性的意义和研究价值[2]。2017年四川省阿坝藏族羌族自治州九寨沟县发生7.0级地震,这次地震使得林地资源和野生动物栖息地遭到了严重损毁,区域内局部范围的生态系统功能衰退,区域生态状况急剧恶化,大熊猫等珍稀野生动物生命安全受到极大威胁。地震导致山体滑坡、泥石流等自然灾害,进一步造成森林植被受到巨大破坏[1, 3]。因此,加速地震灾区的森林生态修复,对保障该区域生态安全、构筑生态屏障具有重要现实意义。
土壤微生物是森林生态系统不可缺少的重要组成部分,在生态系统的养分循环、凋落物分解、土壤肥力的形成与维持、碳氮周转等方面具有不可替代的作用[4, 5]。土壤微生物是对环境变化最敏感的生物指标,是比土壤有机质更可靠的环境变化指标[6]。微生物在森林土壤环境中占有十分重要的位置,量化土壤微生物对于掌握地震灾区森林土壤微生物现状具有十分重要的意义。本研究采用实时荧光定量PCR的方法对九寨沟典型受灾区不同受损程度粗枝云杉(Picea asperata)林土壤微生物丰度进行调查分析,探讨地震对九寨沟粗枝云杉林微生物及其与土壤理化因子之间的相关性,为震后森林土壤生态系统的恢复、重建和协调提供科学依据。
HTML
-
研究区域九寨沟国家级自然保护区,位于青藏高原东缘(E103°46′—104°05′,N32°55′—33°16′;1996−4764 m a.s.l.)。受热带和海洋性季风气候影响,经常暴露在亚冰点温度下,年平均温度为6 ℃−7 ℃,最高温度和最低温度分别为30.7 ℃和−20.2 ℃。年平均降水量约761.8 mm,大部分降水量在5月至8月之间。从10月到次年4月下旬为雪被期。区域内植被丰富,有冷杉(Abies ernestii)、落叶松(Laris potaninii)、华山松(Pinus armandii)、油松(Pinus tabulaeformis)等,林下植被以高山柳(Salix cupularis)、绣线菊(Spiraea salicifolia)等为主[7]。
-
研究地段位于九寨沟在地震中受到严重影响的典型粗枝云杉林,土体有翻动、崩塌、滑坡等现象。在林内分别选择受损严重、受损中等及受损较轻的3个取样样地,编号分别为D1、D2和D3,同时选择没有明显的断裂、颠覆和滑坡等土地破坏现象,土体及土表植被相对完整的土壤作为对照,编号CK。样地具体情况见表1。
研究地Studied sites 经纬度Longitude and latitude 海拔Elevation (m) 受地震影响情况Effect of the earthquake CK E103°44′23″, N33°13′50″ 2692 未遭到地震明显破坏,植被生长良好 D1 E103°44′24″, N33°13′52″ 2698 受地震破坏严重,山体有崩塌,滑坡严重,植被部分被掩埋 D2 E103°43′33″, N33°33′32″ 2676 受地震破环中等,部分土层断裂或土体坍塌,树木有折断、枯萎现象 D3 E103°43′06″, N33°10′56″ 2876 受地震破坏较轻,表层土壤明显松动,有少部分植被破坏 CK:未受损粗枝云杉林Undamaged Picea crassifolia forest; D1:受损严重的粗枝云杉林Severely damaged Picea crassifolia forest; D2:受损中等的粗枝云杉林moderately damaged Picea crassifolia forest; D3:受损较轻的粗枝云杉林slightly damaged Picea crassifolia forest.下同。 Table 1. Basic information of studied sites
-
在4个对比研究点,分别随机选取3个20 m×20 m的样地作为重复采样点。采用5点法,在每个重复采样点采集0−30 cm土样5份,并去除枯枝落叶等,然后将5点土样混合均匀后装入袋子。分别于2019年4月6日(春季)、6月12日(夏季)、9月25日(秋季)和12月2日(冬季)采集土壤样品。样品储存在冷冻箱中,并在24小时内运送至实验室。每个样本分成两份,一份使用真空冷冻干燥机在−80 °C下冷冻干燥(Wizard2.0,VirTis USA),供土壤DNA提取和微生物丰度检测使用。另一份在65 °C下烘箱干燥72小时,然后分析以确定质量(quantity)(表2)。
采样点
Sampling site采样时间
Sampling time检测指标Determined indexes 土壤pH
Soil pH土壤容重
Soil bulk density土壤总孔隙度
Soil total
porosity /%Soil organic carbon
土壤有机碳
(SOC) /(g·kg−1)总氮
Total nitrogen
(TN) /(g·kg−1)总磷
Total phosphorus
(TP) /(mg·kg−1)CK 春季Spring 6.69±0.04 0.84±0.01 57.68±0.99 66.83±1.80 4.41±0.06 920.95±41.55 夏季Summer 6.62±0.08 0.82±0.02 71.34±1.67 48.17±1.66 4.08±0.12 795.04±26.91 秋季Autumn 6.61±0.02 0.82±0.02 67.58±1.31 51.51±1.80 3.35±0.08 678.08±28.65 冬季Winter 6.65±0.03 0.83±0.01 59.67±0.62 58.03±1.78 4.60±0.08 890.61±22.98 D1 春季Spring 6.64±0.02 0.96±0.01 51.01±0.20 48.25±0.29 2.15±0.07 705.30±28.01 夏季Summer 6.61±0.05 0.94±0.03 55.39±0.07 34.63±1.37 2.10±0.12 635.01±25.42 秋季Autumn 6.62±0.02 0.93±0.01 58.52±0.69 33.10±1.76 2.11±0.11 595.72±28.11 冬季Winter 6.66±0.02 0.95±0.01 53.68±1.11 42.27±1.03 2.23±0.10 677.24±23.89 D2 春季Spring 6.65±0.02 0.92±0.02 57.10±1.36 51.63±1.41 2.32±0.13 734.38±23.43 夏季Summer 6.63±0.01 0.89±0.01 61.30±1.20 25.27±1.79 2.13±0.09 654.98±32.52 秋季Autumn 6.62±0.03 0.88±0.02 63.64±1.23 36.00±0.40 2.18±0.06 619.40±10.74 冬季Winter 6.66±0.02 0.88±0.01 56.46±0.50 43.85±0.75 2.35±0.11 639.84±24.29 D3 春季Spring 6.70±0.02 0.87±0.02 57.43±1.32 58.95±1.22 2.95±0.17 788.27±24.21 夏季Summer 6.66±0.02 0.84±0.01 72.65±0.57 43.00±1.70 2.16±0.06 668.92±37.67 秋季Autumn 6.63±0.01 0.83±0.01 67.21±1.24 45.94±1.23 2.22±0.07 645.57±42.60 冬季Winter 6.66±0.01 0.86±0.01 54.59±1.29 53.27±1.07 2.97±0.11 734.71±33.04 Table 2. Physical and chemical characteristics of different sampling sites
-
冻干土壤样品的基因组DNA均采用北京天恩泽基因科技有限公司的Soil DNA out试剂盒并按操作说明提取。并采用天根生化科技(北京)有限公司Universal DNA Purification Kit试剂盒纯化所有的样品DNA,纯化步骤按试剂盒说明书进行。经过琼脂糖凝胶电泳回收纯化后的总DNA 10-100 ng作模板,在Bio-Rad iCycle PCR仪中进行PCR扩增反应。采用338f:5′-(GC)-CCTACGGGAGGCAGCAG-3′和518r:5′-ATTACCGCGGCTGCTGG-3′扩增细菌16S rRNA基因。采用U1:5′-(GC)-GTGAAATTGTTGAAAGGGAA-3′和U2:5′-GACTCCTTGGTCCGTGTT-3′扩增真菌18S rRNA基因[8, 9]。选择凝胶上的条带进行切胶回收和扩增,扩增产物纯化后,连接到pMD19-T载体(TaKaRa)相连,再转入大肠杆菌感受态细胞DH5α在培养皿中进行培养,进行蓝白斑筛选,将获得阳性克隆。选择阳性克隆并提取质粒供qPCR反应的标准曲线使用,质粒浓度采用ScanDrop100超微量核酸蛋白测定仪(Analytik Jena,德国)测定,然后分别进行10倍梯度系列稀释以作为定量PCR扩增的标准品在Mini-Opticon Real-Time system (Bio-Rad, USA)上进行扩增,根据所得标准曲线计算得出样品中的基因拷贝数,最后以每克样品(干重)的基因拷贝数为单位进行分析。PCR反应效率和标准曲线的相关系数分别为:细菌107.5%和r2=0.998,真菌98.3%和r2=0.997。
-
实时荧光定量PCR扩增得到的不同微生物基因拷贝数经过Ln对数转换后进行统计分析,用SPSS 19.0统计软件进行单因素方差分析(One-way ANOVA),多重比较采用最小显著差异法(LSD)。微生物基因丰度与环境因子的相关关系采用Pearson法分析。数据整理、计算与作图均采用Microsoft Excel 2013软件。
2.1. 样地设置
2.2. 样品采集
2.3. 微生物丰度检测
2.4. 数据处理
-
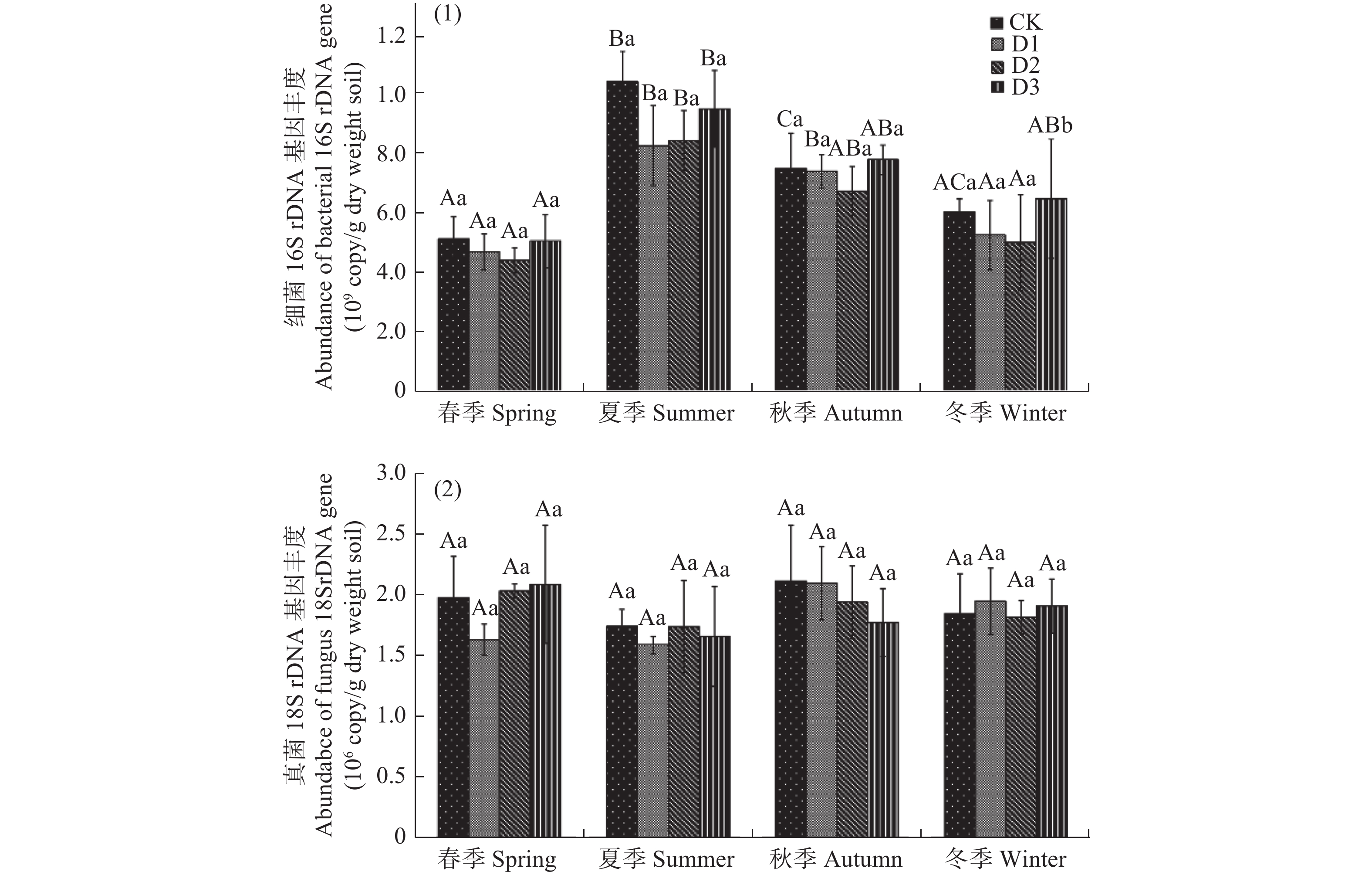
受地震灾害影响各林地土壤细菌16S rDNA和真菌18S rDNA基因检测结果见图1。如图1,受灾地森林土壤细菌16S rDNA基因拷贝数范围为4.39×108−9.58×108 copies/g干土。在相同的采样季节,不同损毁地土壤细菌16S rDNA丰度无显著差异(P>0.05)。春季和夏季,CK土壤细菌16S rDNA丰度最高,D3次之;而秋季和冬季,D3细菌16S rDNA基因丰度最高,CK次之。各损毁林地土壤细菌16S rDNA丰度随时间变化波动较大,表现相似的季节性变化特征。全年各林地细菌16S rDAN基因丰度春季最低,从春季到夏季显著增长(P<0.05),达到全年最高值,从夏季到冬季又显著降低(P<0.05)。
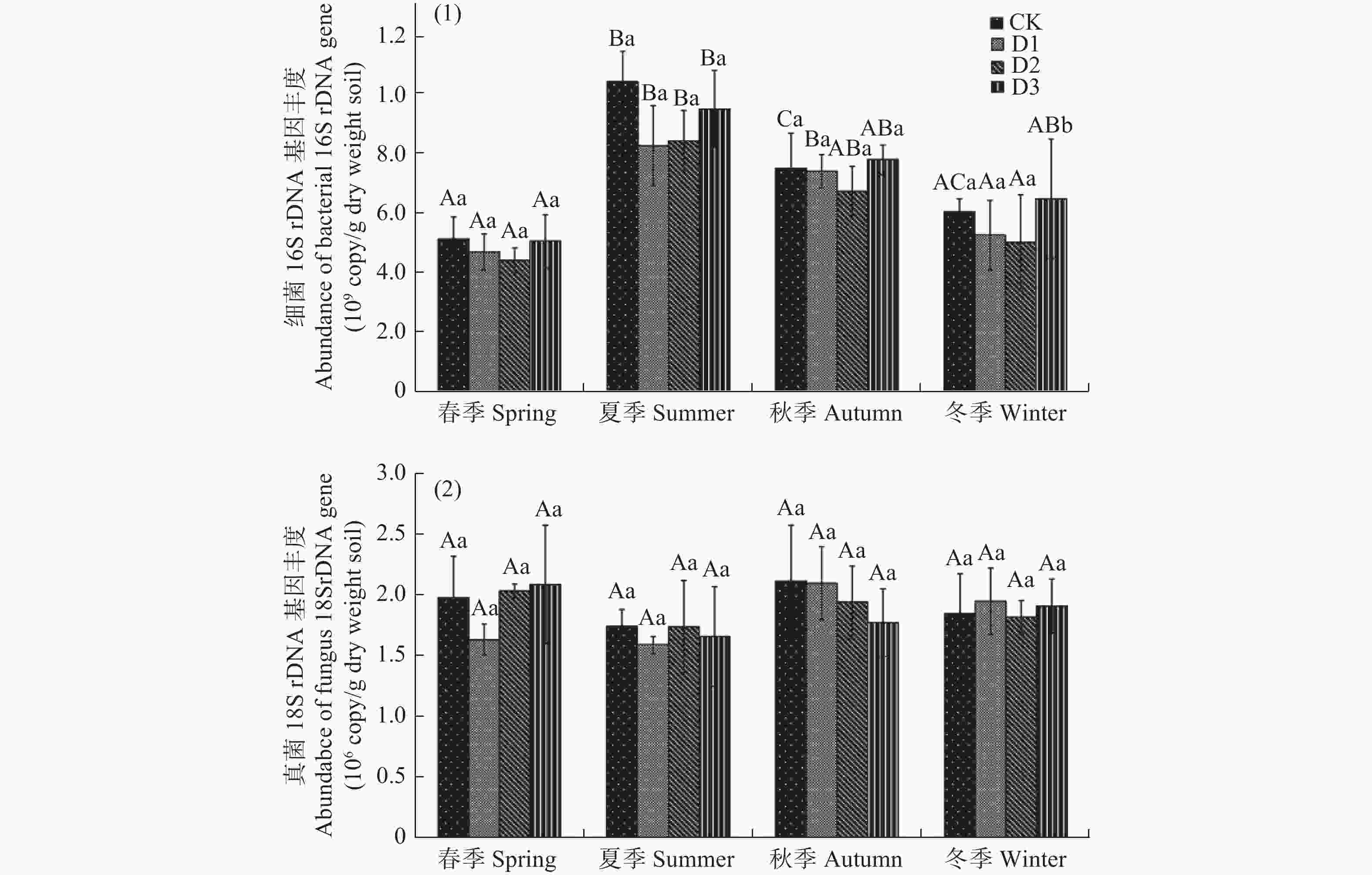
Figure 1. The abundance of bacterial (1) and fungal (2) rDNA genes in forest soils damaged by earthquake in April (spring), June (summer), September (autumn), and December (winter) of 2019
在相同采样季节,各损毁地土壤真菌18S rDNA丰度也无显著的差异性(P>0.05),基因拷贝数范围为1.58×106−2.09×106 copies/g干土(见图1b)。各损毁地土壤真菌18S rDNA丰度季节性变化相似,但与细菌16S rDAN基因不同,且波动不大。全年各采样点真菌18S rDNA丰度从春季到夏季略有降低,但不显著(P>0.05),从夏季到秋冬季又逐渐增加,也不显著(P>0.05)。
-
采用SPSS 19.0软件对各损毁林地土壤中的微生物丰度与相应的环境因子进行Pearson相关性分析,结果见表3,由表3可知,CK土壤细菌16S rDNA基因丰度与土壤pH值(R=−0.617)呈显著负相关性(P<0.05),与土壤总孔隙度(R=0.884)和总氮(R=0.713)分别呈极显著的正相关性(P<0.01)。D1土壤细菌16S rDNA基因丰度与土壤pH值(R=−0.796)呈极显著负相关性(P<0.01),与土壤容重(R=−0.610)呈显著负相关性(P<0.05),并与土壤总孔隙度(R=0.733)和总氮(R=0.585)分别呈极显著的正相关性(P<0.01)和显著的正相关性(P<0.05)。D2土壤细菌16S rDNA基因丰度与土壤总孔隙度(R=0.612)和总氮(R=0.838)分别呈显著的正相关性(P<0.05)和极显著的正相关性(P<0.01);D3土壤细菌16S rDNA基因丰度则与土壤总孔隙度(R=0.746)和总氮(R=0.779)分别呈极显著的正相关性(P<0.01)。D1土壤真菌18S rDNA基因丰度与土壤有机碳(R=0.705)呈显著的正相关性(P<0.05),而D2、D3和CK的土壤真菌18S rDNA基因丰度与所检测的环境因子之间相关性均不显著(P>0.05)。
采样点
Sampling site细菌16S rDNA丰度Bacteria 16S rDNA abundance 土壤pH
Soil pH土壤容重
Soil bulk density土壤总孔隙度
Soil total porosity土壤有机碳
Soil organic carbon总氮
Total nitrogen总磷
Total phosphorusCK 土壤pH Soil pH −0.617* 土壤容重Soil bulk density −0.37 0.428 土壤总孔隙度Soil total porosity 0.884** −0.519 −0.416 土壤有机碳Soil organic carbon −0.255 −0.177 0.026 0.014 总氮Total nitrogen 0.713** −0.557 −0.665* 0.791** 0.107 总磷Total phosphorus 0.209 −0.57 −0.305 0.349 0.693* 0.579* Fungal 18S rDNA abundance真菌18S rDNA丰度 −0.077 −0.148 0.293 −0.117 0.314 −0.078 0.172 D1 土壤pH Soil pH −0.796** 土壤容重Soil bulk density −0.610* 0.623* 土壤总孔隙度Soil total porosity 0.733** −0.489 −0.511 土壤有机碳Soil organic carbon 0.129 0.011 −0.268 0.734** 总氮Total nitrogen 0.585* −0.318 −0.532 0.620* 0.221 总磷Total phosphorus 0.324 −0.212 −0.552 0.711** 0.813** 0.312 Fungal 18S rDNA abundance真菌18S rDNA丰度 0.075 0.151 −0.142 0.477 0.705* 0.153 0.495 D2 土壤pH Soil pH −0.366 土壤容重Soil bulk density −0.256 0.612* 土壤总孔隙度Soil total porosity 0.612* −0.760** −0.516 土壤有机碳Soil organic carbon −0.038 −0.212 −0.569 0.419 总氮Total nitrogen 0.838** −0.398 −0.5 0.698* 0.35 总磷Total phosphorus 0.191 −0.348 −0.547 0.47 0.827** 0.48 Fungal 18S rDNA abundance真菌18S rDNA丰度 −0.14 0.123 0.416 −0.039 0.08 −0.085 0.081 D3 土壤pH Soil pH −0.433 土壤容重Soil bulk density −0.552 0.689* 土壤总孔隙度Soil total porosity 0.746** −0.361 −.682* 土壤有机碳Soil organic carbon −0.102 −0.675* −0.343 −0.181 总氮Total nitrogen 0.779** −0.484 −0.56 0.798** 0.152 总磷Total phosphorus 0.06 −0.726** −0.434 0.054 0.803** 0.16 Fungal 18S rDNA abundance真菌18S rDNA丰度 −0.56 0.535 0.507 −0.422 0.017 −0.228 −0.019 *, *相关系数在0.05水平上显著Correlation is significant at the 0.05 level,**, 相关系数在0.01水平上显著Correlation is significant at the 0.01 level Table 3. Pearson’s correlation analyses between soil microbial gene abundance and environmental variables at each sampling site
3.1. 不同损毁地微生物的季节性变化特征
3.2. 微生物丰度与环境因子的相关性
-
微生物rDNA基因的季节性变化结果表明,九寨沟粗枝云杉人林受地震不同损毁地土壤细菌和真菌rDNA基因丰度均具有明显的动态特征(见图3)。与地震前其他高海拔生态环境中的研究结构相同,季节性变化对微生物的影响具有明显的阶段性特征,亚高山/高山森林的微生物群落从冬季到夏季可能会增加[10-12],本研究中各损毁林地土壤细菌从春季到夏季表现为显著增长,到秋冬季节又显著降低,且冬季高于春季。而与此相反,真菌则是从春季到夏季表现为降低趋势,夏季到秋冬季节又缓慢增长。这些结果都反映了高寒森林季节性变换对微生物产生的不同影响,而且由于人工林不同的发育阶段所代表的区域性环境因子对微生物也产生了不同的影响。春季充沛的降雨和土壤冻融带来的融雪可能引起细胞内外水势的严重失衡[13],从而导致微生物,尤其是细菌的死亡。由于对低温具有较强的耐受性,以及菌丝体和孢子的形成,大多数真菌能适应土壤冻融过程中快速变化的极端条件,因此真菌丰度随季节性的变化幅度没有细菌剧烈。另外,由于高山森林凋落物的年峰值一般出现在深秋和初冬,新鲜凋落物相对容易分解[14],丰富的凋落物增加了大量微生物胞外酶的产生[15, 16]。当冬季积雪覆盖时,相对稳定的环境也有助于维持微生物活性,促进有机质分解,平衡土壤水分,因此秋冬季节微生物丰度维持在比春季更高的水平。
-
由于地震对土壤和森林植被的破坏造成了土壤养分的明显下降,土壤黏粒的迅速流失,使土壤微生物失去了繁育生长所需的营养物质和生存载体[17]。土壤微生物对外界环境的变化又十分敏感,这就导致了土壤微生物丰度的下降。由于地震破坏程度的不同,林地土壤中资源的可利用性差异也不相同,不同损毁地微生物下降的趋势也就表现相应的差异。受损较轻的D3林地较受损严重的D2和D3林地,植被覆盖和生物量相对较高,微生物的生存空间更大,食物来源更广,微生物的生长繁殖优势相对也就更大,因此表现为丰度降低程度相对较小。
研究发现土壤理化指标和微生物丰度受地震影响明显下降,而且在不同损毁地表现出一定的影响差异。微生物的生长活性常常受氮有效性的限制,环境中氮有效性越高,微生物的活性越强,繁殖也越快[18]。在不同损毁林地细菌丰度均与土壤总氮呈显著或极显著的正相关性,充分表明了,地震带来的土壤氮素的流失可能是引起土壤微生物丰度变化的重要因素。另一个重要的因素是土壤总孔隙度,它对各损毁林地的影响都显著。土壤孔隙结构在土壤中的分布呈现出高度的异质性,并且极易受周围环境的影响,能及时反映土壤涵养水源、吸持水分及保障气体畅通能力。同时土壤孔隙特征是影响微生物功能、活性和异质性的主要因素[19]。地震对土壤总孔隙度造成不同程度的严重影响[20],使土壤总孔隙度显著降低,随之表现为土壤微生物相应的显著降低。
综上所述,受地震灾害影响九寨沟粗枝云杉林土壤细菌16S rDNA和真菌18S rDNA基因丰度均有不同程度的降低;与相似生境研究结果一致,微生物丰度的变化具有明显的季节性特征,但细菌和真菌丰度的变化趋势不同。各林地土壤微生物丰度受到环境因子的影响,其中土壤pH值、土壤总孔隙度、土壤总氮、土壤容重对土壤微生物产生的影响更深刻。


















 DownLoad:
DownLoad: