-
镉是剧毒重金属元素之一,易通过生物链在人体富集,最终可能会影响神经、生殖系统并导致癌症[1]。据我国前期调查,镉污染土壤点位超标率达到7.0%,污染情况不容乐观,城市土壤受到的Cd污染尤为突出[2-3]。由于应用成本低、环境友好等优点,以特定植物降低重金属污染的植物修复技术,近年来受到了广泛的关注[4-5]。尤其是在受污染的城市地区,兼具景观营造和生态修复的观赏植物正成为修复重金属污染土壤的关注热点[6]。
木芙蓉(Hibiscus mutabilis L.)是锦葵科 (Malvaceae)木槿属 (Hibiscus)落叶灌木或小乔木,花姿优美,为秋季优良的园林观赏树种,在四川等多地均有园林应用[7]。研究发现木芙蓉对重金属Pb具有较强耐性和富集能力,可作为修复轻度、中度Pb污染土壤的树种之一[8],但目前对于木芙蓉的Cd胁迫响应机制还鲜见文献报道。有研究表明木槿属植物木槿(Hibiscus syriacus L.)[9]、海滨木槿(Hibiscus hamabo Sieb. & Zucc.)[10]、红麻(Hibiscus cannabinus L.)[11]具有较强的Cd耐性和富集能力,推测木芙蓉可能也具有相似的能力。目前的研究更注重高浓度Cd对植物的毒害作用,对低浓度Cd下的植物响应较少关注,这与城市土壤低浓度Cd污染分布更广不符[3]。因此,以4个木芙蓉品种(‘百日华彩’、‘彩霞’、‘醉红’、‘单瓣红’)为对象,探讨不同低浓度Cd处理下(0,2.5,5,10 mg·L-1)木芙蓉的耐性和积累特性,以期为将木芙蓉作为兼具景观营造和生态修复的观赏植物应用于Cd污染的城市土壤中提供理论依据。
-
供试植物:木芙蓉,4个品种为‘百日华彩’(BRHC)、‘彩霞’(CX)、‘醉红’(ZH)、‘单瓣红’(DBH)。均为长势一致的当年生扦插苗,由成都市植物园(成都市公园城市植物科学研究院)提供,经检验扦插苗含镉量均为0。
将选定的扦插苗进行重剪,控制株高和根长基本一致,移栽至装有10 L 1/2 Hoagland营养液的20孔实验盆(共4个品种,每个品种5株),共4盆。放置在光照培养室中进行预培养(设置为恒温25 ℃,光照16 h,黑暗8 h),每隔24小时通过称重法补充蒸发的水分,以维持溶液中相对恒定的Cd浓度,同时每5 d更换一次营养液,预培养15 d后,进行Cd处理实验。
-
采用水培试验, 设置4个Cd(CdCl2·2.5 H2O,分析纯)处理浓度:0(CK)、2.5 mg·L−1(Cd2.5)、5 mg·L−1(Cd5)、10 mg·L−1(Cd10),每个处理重复5次,完全随机排列。
-
于Cd处理15天后采样,将根部置于20 mmol·L−1 Na2-EDTA中浸泡15 min后,经自来水洗净,用去离子水润洗、擦干,分为根和地上部,取部分叶片用于叶绿素含量的测定,剩余部分置于105℃条件下杀青30 min、75 ℃条件下烘干至恒重,称重、粉碎后用于Cd含量的测定。
-
生长指标:用直尺测量根长、新枝长,根长为整株的最长根长,新枝长为整株的最长枝长度。
生物量:用称重法测定干重。
叶片色素:叶绿素a(Chl a)、叶绿素b(Chl b)、总叶绿素(Chl )与类胡萝卜素(Car)的测定采用王学奎的方法(紫外可见分光光度计UVmini-1280)[12]。称取0.1 g叶片,置于10mL容量瓶内,加95%乙醇定容至10 mL,摇匀置于避光处,过夜。待第2天残渣完全变白后,分别在665、649和470 nm测定吸光值。按如下公式计算各色素含量:
Chl a (mg/g FW) = 13.95A665 − 6.88A649 × 10/(1000 × W)(W为叶片鲜重,下同)
Chl b (mg/g FW) = 24.96A649 − 7.32A665 × 10/(1000 × W)
Chl (mg/g FW)= Chl a+Chl b
Car (mg/g FW)=(1000A470-2.05 Chl a-114.8 Chl b)×10/(245×1000×W)
Cd含量:采用微波消解-原子吸收分光光度计法(微波消解仪MDS-6G、赶酸器TK12、原子吸收光谱仪AA-7020)[13]。准确称取0.5000 g样品于聚四氟乙烯消解罐中,加入6 mL硝酸,混匀放置2 h以进行冷消化,消化前再加入2 mL H2O2。设置微波消解系统的升温条件进行消化,消解完成后移至赶酸仪上开盖赶酸,冷却后用去离子水定量转移并定容至25 mL,上机测定。
-
耐性指数(Tolerance Index,TI):Cd处理下生物量/CK生物量[14];镉积累量=镉含量*生物量;富集系数(Bioconcentration Factor, BCF)=植株根Cd含量/溶液Cd含量[15];转运系数(Transfer Factor, TF)= 植株地上部Cd含量/植株根Cd含量[16]。
采用 SPSS 22.0进行统计分析,选择最小显著差异法(Least Significant Difference, LSD)进行多重比较,图表制作采用Origin 9.0 和Excel 2016。
-
如表1所示,不同浓度Cd处理下,4个品种木芙蓉生长差异明显,随着Cd浓度增加表现为低浓度(Cd2.5)促进生长,高浓度(Cd10)抑制生长。其中,在Cd2.5处理时,‘百日华彩’的生物量相对CK增加最多,根部和地上部生物量分别增加了25.00%和16.64%,根长、新枝长也较其余品种增加最多;在Cd10处理下,‘单瓣红’的生物量下降幅度最大,根部和地上部生物量分别降低21.43%和24.44%,而‘百日华彩’的生长受抑制程度最小,根部生物量降低19.44%,地上部生物量增加了1.31%,说明Cd处理下‘百日华彩’的生长相对其他品种更好。Cd处理下,木芙蓉TI值为0.76~1.17;随着Cd浓度增加,TI值降低;不同品种的TI值大小为‘百日华彩’>‘彩霞’>‘醉红’>‘单瓣红’,其中,‘百日华彩’在所有Cd浓度处理下的TI值均大于1,表明其对Cd的耐性最强,而‘单瓣红’在Cd5时TI值已小于1,在Cd10时仅为0.76,表明其对Cd相对敏感。
品种 处理 根长/cm 新枝长/cm 根部生物量/g 地上部生物量/g 总生物量/g 耐性指数TI 百日华彩 CK 13.90±0.82Bb 7.07±0.12Ca 0.36±0.04Ba 6.13±0.03Ba 6.49±0.01Ba − Cd2.5 16.40±1.82Ab 11.53±1.11Aa 0.45±0.02Ba 7.15±0.19Aa 7.6±0.2Aa 1.17±0.03Aa Cd5 17.43±1.50Ab 8.43±0.21Ba 0.53±0.06Aa 6.45±0.3Ba 6.98±0.26ABa 1.08±0.04Aa Cd10 13.27±0.12Bb 7.13±0.12Ca 0.29±0.03Ca 6.21±0.24Ba 6.5±0.27Ba 1.00±0.04Aa 彩霞 CK 16.4±1.22Ca 5.10±0.44Bb 0.34±0.05BCa 5.38±0.58ABb 5.72±0.58Bb − Cd2.5 18.37±0.59ABa 7.37±0.61Ac 0.38±0.04Ba 6.03±0.63Ab 6.41±0.63Ab 1.12±0.05Aa Cd5 19.43±0.95Aa 7.40±0.53Ab 0.46±0.01Ab 5.66±0.44ABb 6.12±0.44ABb 1.08±0.14Aa Cd10 16.93±0.61BCa 6.60±0.20Aa 0.29±0.02Ca 5.33±0.33Bb 5.62±0.35Bb 0.99±0.15Aa 醉红 CK 13.8±0.95Bb 7.37±0.67Ca 0.28±0.02Ab 5.93±0.28ABab 6.21±0.26ABab − Cd2.5 18.47±0.23Aa 9.60±0.35Ab 0.24±0.01Ab 6.32±0.53Ab 6.56±0.52Ab 1.06±0.09Aa Cd5 18.00±1.64Aab 8.23±0.06Ba 0.26±0.03Ac 6.02±0.46ABab 6.29±0.46ABb 1.02±0.12Aa Cd10 12.27±1.6Bb 5.33±0.45Db 0.24±0.03Ab 5.66±0.04Bab 5.9±0.01Bab 0.95±0.04Aa 单瓣红 CK 8.57±0.35Bc 4.87±0.21BCb 0.14±0.01Ac 5.85±0.55ABab 5.99±0.55ABab − Cd2.5 10.63±0.32Ac 6.37±0.15Ad 0.17±0.01Ac 6.2±0.28Ab 6.37±0.27Ab 1.07±0.14Aa Cd5 7.40±0.10BCc 5.50±0.70Bc 0.14±0.02Ad 5.54±0.41Bb 5.68±0.39Bb 0.96±0.14Aa Cd10 6.47±0.06Cc 4.43±0.50Cc 0.11±0.01Ac 4.42±0.37Cc 4.53±0.36Cc 0.76±0.11Bb 注:表中不同大写字母表示同一品种不同处理间差异显著(P < 0.05),不同小写字母表示同一处理条件下不同品种之间差异显著(P < 0.05),短横线(−)表示无值。
Note: In the table, different capital letters indicate significant difference among the four treatments (CK, Cd2.5, Cd5 and Cd10) for the same variety (P < 0.05). Different lowercase letters indicate significant difference between different varieties under the same treatment (P < 0.05). The short line (−) indicate no values.Table 1. Growth traits and relative tolerance index of different Hibiscus mutabilis varieties under Cd treatment
-
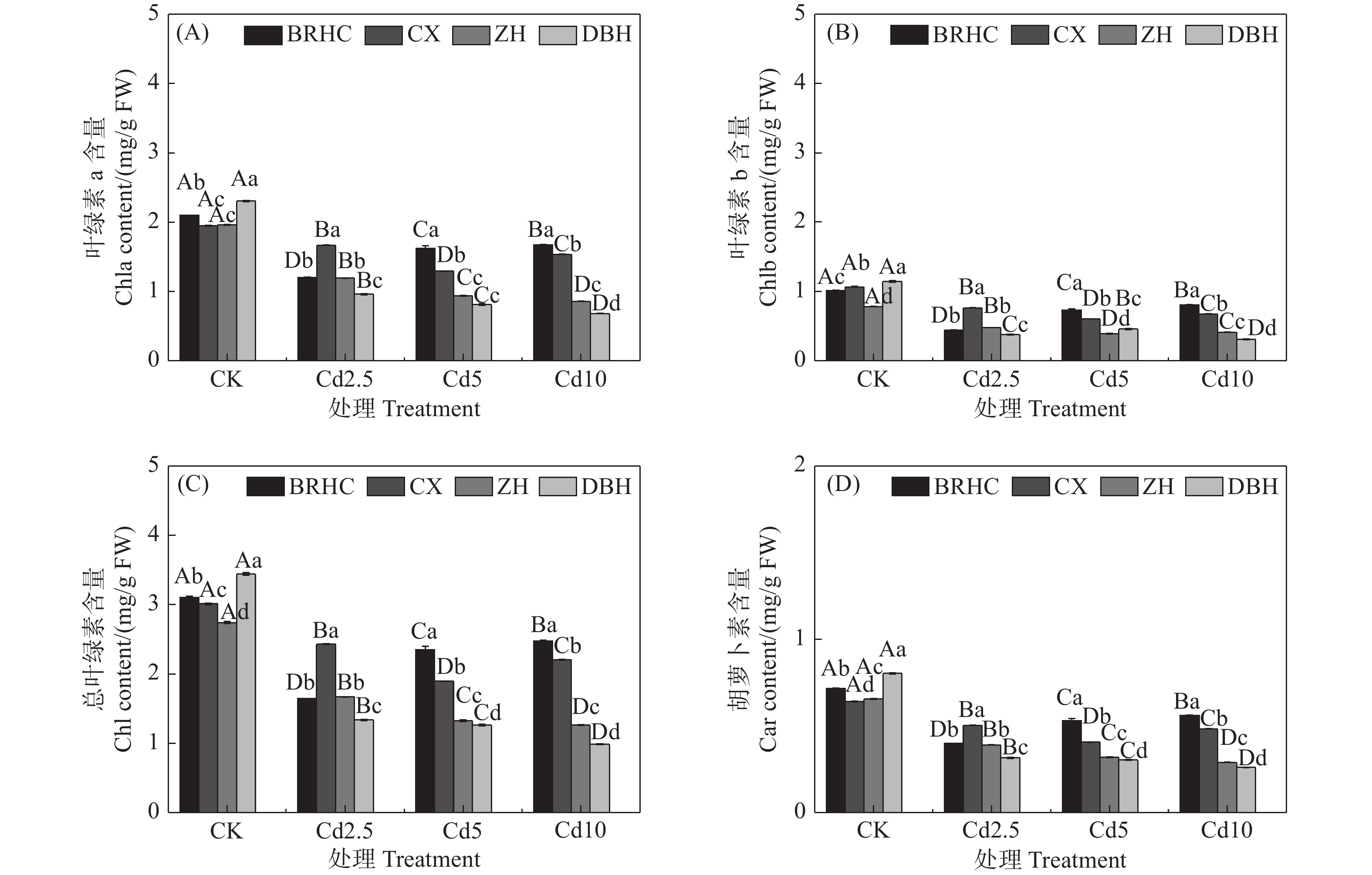
Cd处理下,四个品种木芙蓉的叶绿素a、叶绿素b、总叶绿素和类胡萝卜素相对CK均显著降低(见图1),但不同品种降低程度不同,Cd处理下叶片色素相对CK下降幅度表现为:‘百日华彩’<‘彩霞’<‘醉红’<‘单瓣红’。植物叶片色素的高低能间接反映植物对Cd的耐受性,在Cd10处理时,‘百日华彩’的总叶绿素和类胡萝卜素分别是其余品种的1.12~2.52倍和1.16~2.17倍,说明‘百日华彩’对Cd的耐性最强。
-
由图2可知,4个木芙蓉品种根部和地上部Cd含量随着Cd浓度的增加而增加,且根部Cd含量远高于地上部,分别为205.01~1089.35 mg·kg−1和27.70~188.92 mg·kg−1,根部Cd含量是地上部的2.60~13.32倍。在各Cd浓度条件下,‘百日华彩’根Cd含量最高,是其余品种的1.08~1.92倍,Cd10时‘百日华彩’根Cd含量最高,达1089.35mg·kg−1;‘彩霞’地上部Cd含量显著高于其余三个品种,是其余品种的1.21~4.16倍,Cd10时达188.92mg·kg−1。
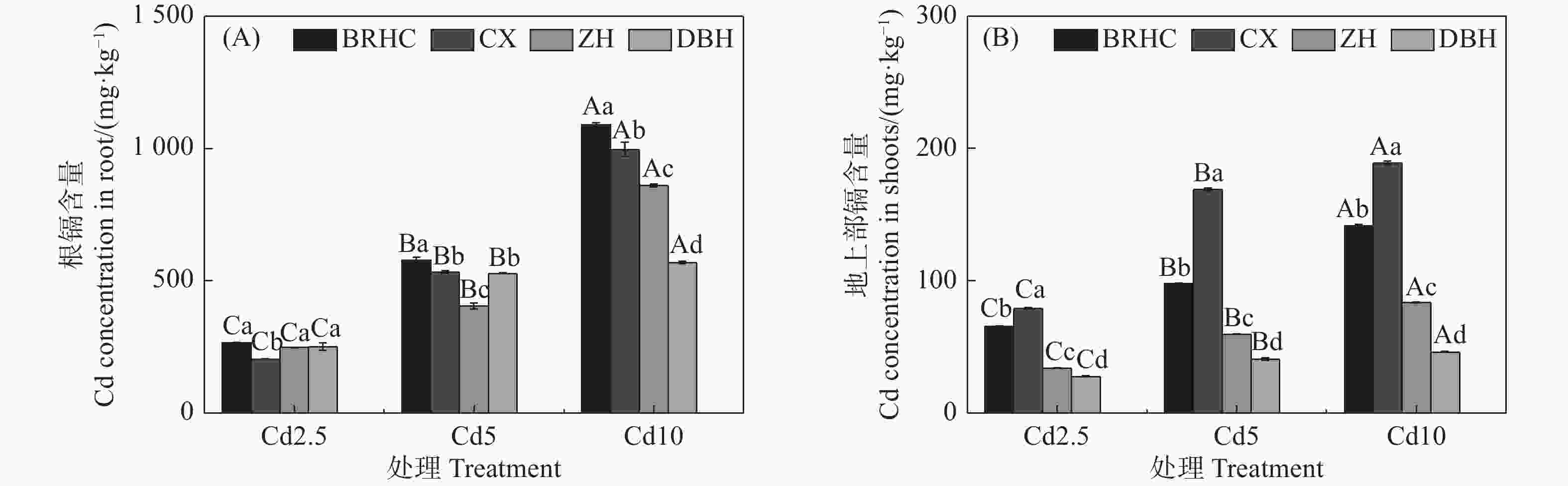
Figure 2. Cd content in the roots (A) and shoots (B) of different Hibiscus mutabilis varieties under cadmium treatment
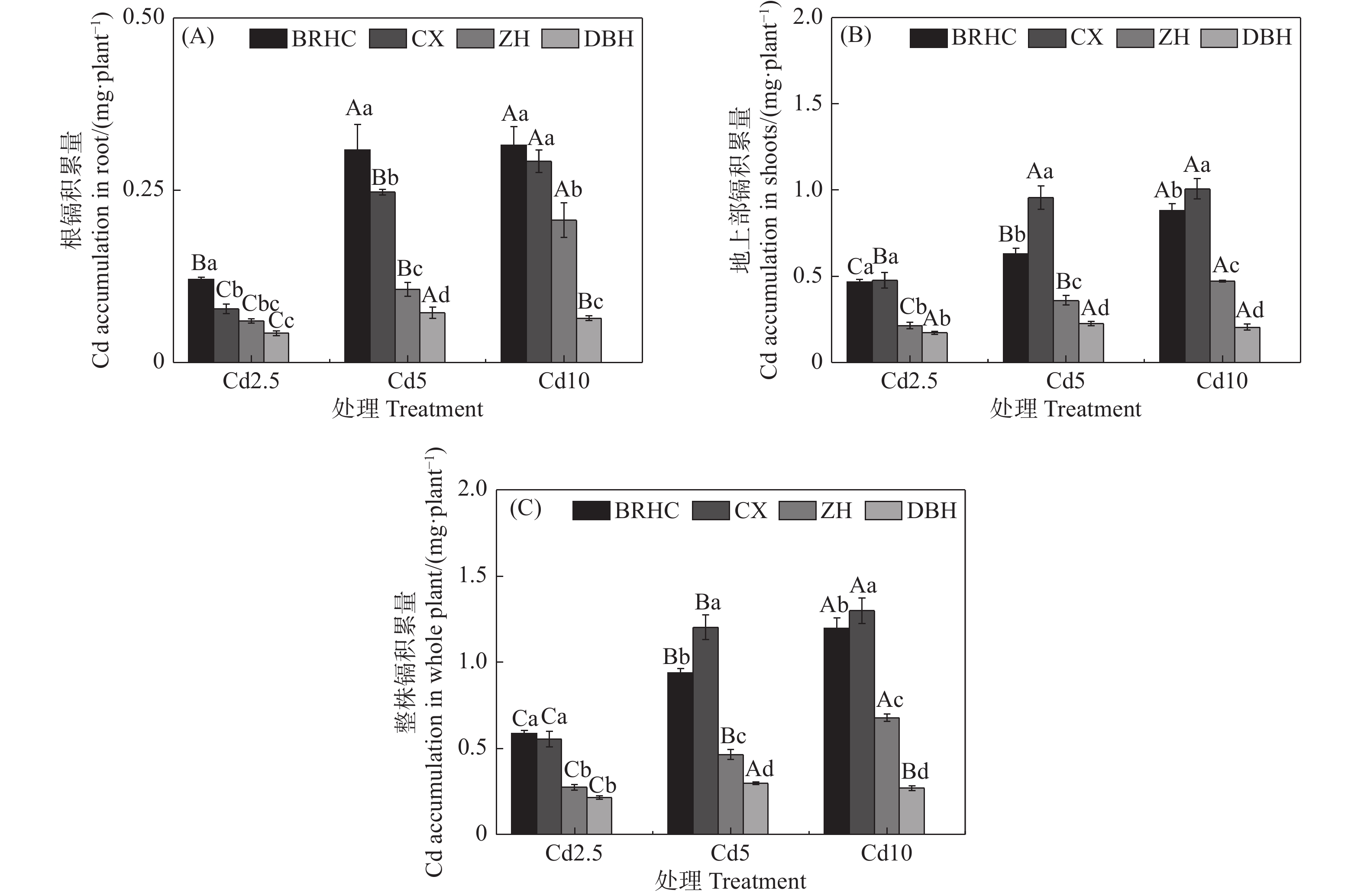
从图3可以看出,随着Cd处理浓度增加,木芙蓉根部和地上部Cd积累量显著增加,地上部Cd积累量显著高于根部,分别为0.17~1.01 mg·plant−1和0.04~0.32 mg·plant−1,地上部Cd积累量是根部的2.04~6.10倍,这主要是由于木芙蓉地上部的生物量远大于根部; ‘百日华彩’的根部Cd积累量显著高于其他三个品种,而‘彩霞’的地上部Cd积累量最高;不同品种木芙蓉整株Cd积累量差异显著,为0.21~1.30 mg·plant−1,品种间大小为:‘彩霞’>‘百日华彩’>‘醉红’>‘单瓣红’,‘彩霞’的整株Cd积累量分别为其余三个品种的1.00~4.49倍,Cd10时,‘彩霞’的整株Cd积累量最高,为1.30 mg·plant−1。

Figure 3. Cd accumulation in the roots (A) 、shoots (B) and whole plant (C) of
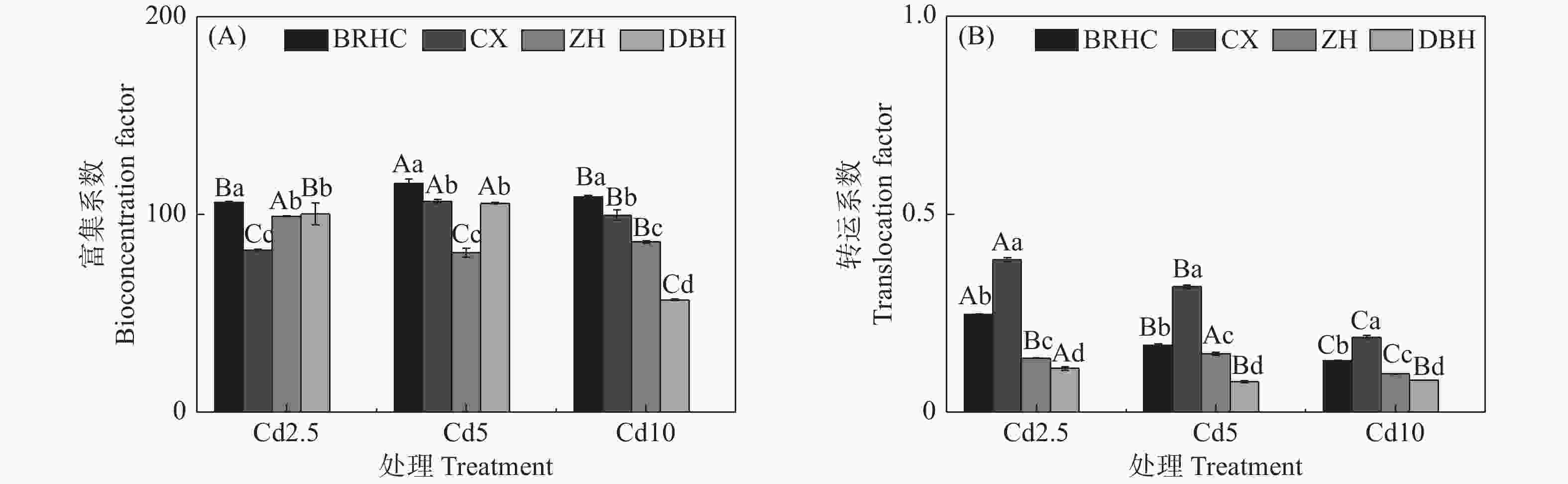
different Hibiscus mutabilis varieties under cadmium treatment 由图4所示,Cd处理下4个木芙蓉品种根部对Cd的富集系数(BCF)为56.83~115.59,其中,‘百日华彩’的富集能力最强,在各Cd浓度下均高于100,为其余三个品种的1.07~1.92倍,在Cd5浓度处理时‘百日华彩’根的富集系数最高,达115.59;Cd处理下木芙蓉的转运系数(TF)为0.08~0.38,随着Cd处理浓度的增加4个木芙蓉品种根部向地上部的转运系数逐渐下降;不同品种的转运系数在各Cd处理浓度下差异显著,大小表现为:‘彩霞’>‘百日华彩’>‘醉红’>‘单瓣红’,‘彩霞’的Cd转运系数最大,是其余品种的1.46~4.12倍。
-
Cd是环境中最有毒的金属之一,直接影响植物的生长并干扰其生理生化过程[1]。木芙蓉的生长随Cd浓度表现为低促高抑,总生物量、根长和新枝长在低Cd浓度处理下相对CK增加,而在高Cd浓度下降低,这与之前的一些研究结果一致:低Cd浓度可以促进植物的生长[17-18],而高浓度的Cd会使植物生长受到显著抑制[19],低cd胁迫下促进植物生长可能与根中葡糖芸苔素转化为生长素有关[20],高Cd胁迫下的根系生长抑制可能是由于细胞壁木质化,限制了细胞扩展和养分吸收[21],而地上部分的响应则可能是由于光合反应受到抑制,从而阻止了有机物质的积累[22]。植物受到这种低剂量促进和高剂量抑制的现象被研究者们称为毒物兴奋效应[23]。Tang等人研究了我国86个城市土壤Cd污染,发现70 %以上的城市Cd污染为轻度及以下[3]。因此利用木芙蓉对Cd的毒物兴奋效应,研究其对低浓度 Cd的响应具有重要的现实意义。
光合作用是植物生长和生物量生产的重要过程,Cd胁迫通常会降低植物的叶绿素含量,从而抑制光合作用[24-25]。Cd对植物中的叶绿素水平的影响可能主要包括两个方面:一方面,由于离子竞争抑制了叶绿素生物合成所必需的Cu2+、Mn2+、Fe2+和Zn2+等离子的吸收,从而导致叶绿素含量降低[26];另一方面,Cd诱导参与叶绿素合成的酶蛋白功能紊乱也是叶绿素含量下降的机制之一[27]。但也有研究表明,有的超积累植物即使暴露在高浓度Cd环境中叶绿素合成也不会受到影响,如马缨丹在200 mg·kg−1的Cd浓度下叶绿素含量也未减少[28]。叶片色素含量通常还被用作评估植物不同品种耐受性的指标[29],Cd处理下‘百日华彩’的叶片色素高于其余木芙蓉品种,表明‘百日华彩’对Cd耐性最强。此外,当选择用于重金属修复的植物时,TI值可直接表征其对于重金属的耐性[30-31],研究表明植物的Cd耐受性与品种有关,Wang等人以TI值为标准将耐受性分为三类:TI>0.6为高耐性,TI=0.35~0.60为中耐性,TI<0.35为敏感型[32]。参考这一耐受性等级,4个供试木芙蓉品种在3种Cd浓度下的TI为0.76~1.17,均大于0.6,表明木芙蓉对Cd具有较高的耐受性,其中‘百日华彩’在所有Cd浓度下TI值均大于1,对Cd的耐性最强。
-
随着Cd处理浓度的增加,木芙蓉的Cd含量升高,且木芙蓉根部Cd含量显著高于地上部,在Cd胁迫浓度为10 mg·kg−1时,地上部Cd含量最高为188.92 mg·kg−1,而根部也达最高值1089.35mg·kg−1,说明木芙蓉具有一定的Cd污染修复潜力。Cd转运系数为0.08~0.38,并呈现出随Cd处理浓度增加而下降的现象,这与海滨木槿、金银花和杞柳对Cd的各部位累积分布大致相同[10, 33-34]。说明木芙蓉在Cd胁迫下通过某种机制限制了Cd向地上部的转移,且随着Cd胁迫的增加而加强,这可能是由于高浓度的Cd破坏了木芙蓉根-茎之间的运输[11]。
4个木芙蓉品种的Cd含量和积累量存在显著差异,‘百日华彩’和‘彩霞’显著高于‘醉红’和‘单瓣红’,这种品种之间对Cd的差异在植物中被证明是由多种机制共同作用导致的:在生理层面上,有研究者提出植物不同品种的Cd积累差异可能与其根系形态有关,如低积累甘薯品种总根长和比根长比高积累品种短[35];Su 等发现不同品种的花生Cd含量的差异主要源于木质部的转运能力[36],在分子层面上,研究者们发现将PjMT1和PjMT2基因转移到烟草植株上,转基因烟草植株Cd积累量明显高于野生型烟草植株[37],研究表明,木芙蓉不同品种表现出对Cd耐性和积累的显著差异,但相关机理还有待进一步深入研究。
-
木芙蓉原产于中国,在东亚及东南亚地区均有分布,迄今已有上千年的栽植历史,四川湖南等地广为栽培,具有极高的文化价值、园艺价值和经济价值,同时木芙蓉还具有较强的固碳、释氧、降温的能力,在城市园林中具有重要的应用价值[38-39]。目前筛选用于植物修复的观赏植物需具有以下优点:1)不会进入食物链,危害人类健康;2)分布面积大,种植季节多,栽培范围广,吸收能力强;3)生长期短,生物量大;4)在其栽培和管理方面有丰富的实践经验,增加其在植物修复中应用的可行性;5)在修复土壤的同时,它们还可以增加城市绿化和美化环境,同时创造经济价值[40-41]。木芙蓉具有上述用于植物修复的观赏植物的特点,其还对Cd具有较强的耐性和积累能力,其中木芙蓉品种‘百日华彩’的 Cd耐性和Cd富集能力最强,作为Cd修复观赏植物的优势最大。因此,在城市Cd污染环境中种植木芙蓉,一方面可以保持其良好的生长状态美化环境,另一方面还可以稳定、持续地吸收积累污染环境中的 Cd以净化土壤,这对于城市地区 Cd污染土壤的修复具有重要的现实意义。
Response of different Hibiscus mutabilis L. varieties to cadmium stress
doi: 10.12172/202303300001
- Received Date: 2023-03-30
- Available Online: 2023-10-24
- Publish Date: 2024-02-25
-
Key words:
- cadmium /
- Hibiscus mutabilis L. /
- varieties /
- tolerance /
- accumulation
Abstract: In order to understand the response of different Hibiscus mutabilis L. varieties to cadmium (Cd) stress, the hydroponic experiments were conducted to investigate the growth, tolerance, leaf pigment, Cadmium (Cd) concentration, Cd accumulation, bioconcentration factor and transfer factor of four different Hibiscus mutabilis L. varieties under different Cd concentrations (0, 2.5, 5, 10 mg·L−1). The results showed that with the increase of Cd concentration, the growth of Hibiscus mutabilis L. showed a toxic hormetic effects. Basically, compared with CK (0 mg·L−1), adding Cd (treatments) increased biomass, root length and new branch length for all Hibiscus mutabilis L. varieties under low Cd concentration, but had opposite effects under high Cd concentration. Under Cd treatment, the leaf pigment concentration of all varieties were significantly lower compared with CK, and the leaf pigment concentration of ‘Bairihuacai’ species was the highest. The Cd tolerance index of Hibiscus mutabilis L. was 0.76-1.17, and ‘Bairihuacai’ species had the highest Cd tolerance index (1.00~1.17). The Cd concentration in the root of Hibiscus mutabilis L. (205.01~1089.35 mg·kg−1) was significantly higher than that in shoot (27.70~188.92 mg·kg−1). The Cd concentration in the root of ‘Bairihuacai’ species was the highest than others, and ‘Caixia’ species had the highest Cd concentration in the aboveground part. Cd accumulation in the aboveground part of Hibiscus mutabilis L. was significantly higher than that in the root, which was 0.17~1.01 mg·plant−1 and 0.04~0.32 mg·plant−1 respectively. The enrichment coefficient of Cd in Hibiscus mutabilis L. was 56.83-115.59, and the transport coefficient was from 0.08 to 0.38. Among which ‘Bairihuacai’ species had the highest Cd enrichment ability, and ‘Caixia’ species had the highest Cd transport coefficient. In conclusion, Hibiscus mutabilis L. has strong tolerance and accumulation ability under Cd pollution condition, among which 'Bairihuacai' species had the best tolerance and the strongest Cd accumulation ability among the four species, and had the greatest potential as an ornamental emediation plants for Cd restoration.



















 DownLoad:
DownLoad: